- Expand your portfolio (e.g. analysis of microorganisms, prenatal diagnostics) with our innovative microfluidic diagnostic platform and open up new target markets in the long term.
- Reduce workload and simplify the operation of your solution thanks to flexible and automated integration of sample preparation inside or outside your solution.
- Strengthen your innovative power and bundle resources where they can unleash their efficiency: Through long-term joint further development of projects, we support you with our expertise from the project idea onwards, including risk analysis.
Liquid Biopsy - Microfluidic concepts for the isolation of biomarkers
Most frequently asked questions and answers:
Are first clinical studies already underway?
Together with clinical partners, we have already been able to examine several tumor samples and conduct the first promising follow-up examinations of isolated tumor cells. We have also been able to automatically isolate and analyze exosomes from urine of prostate cancer patients. We are very interested in a large-scale clinical study - please contact us!
How robust is the automated process compared to the manual method?
With our automated process, we have achieved a robust protocol with a comparable isolation efficiency. *
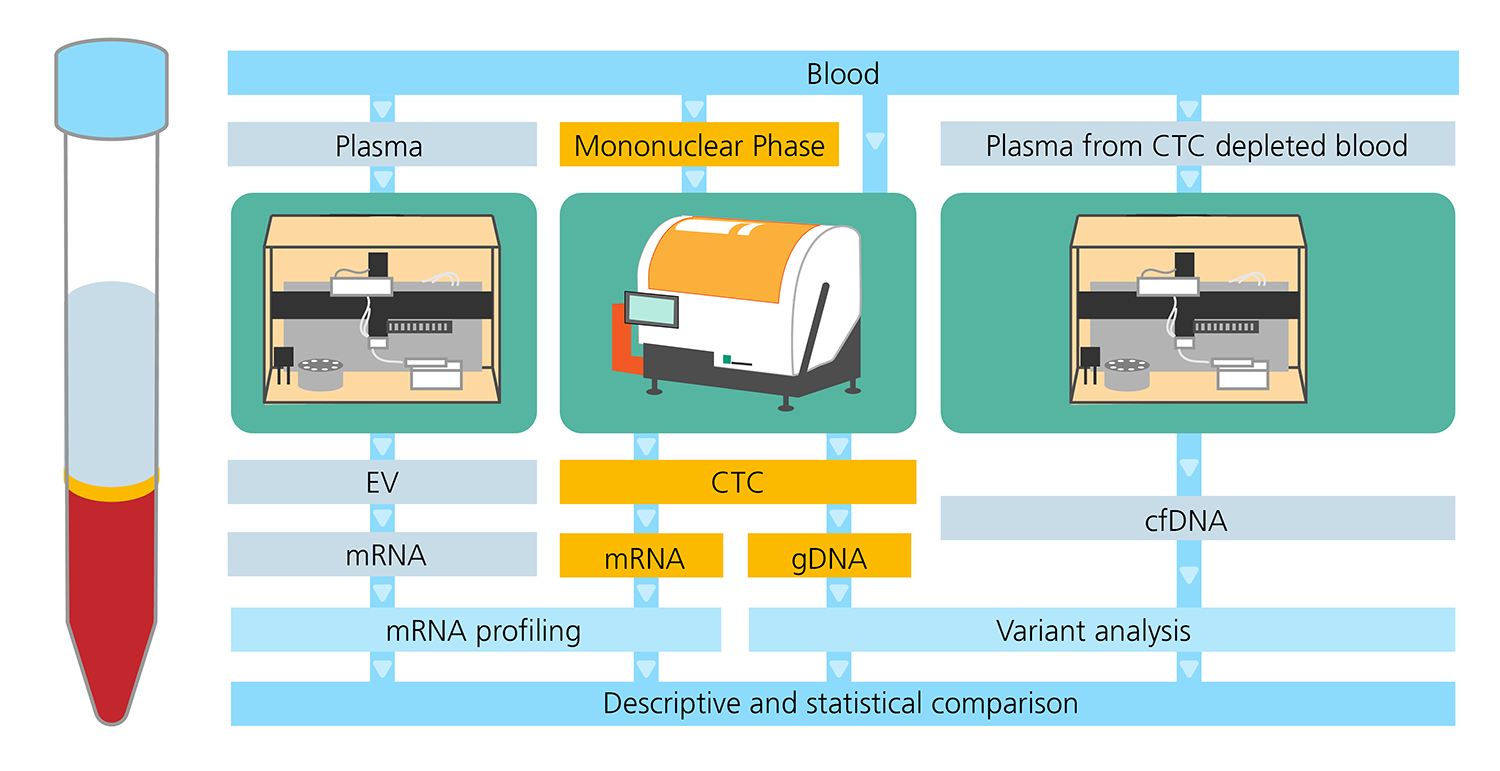
- The CTCelect liquid biopsy platform was developed to realize fully automated enrichment and single cell dispensing of CTCs from whole blood without pre-processing. We optimized each process step with two different carcinoma cell lines with a standard deviation of approximately 10 %, demonstrating up to 87 % enrichment (n = 10) with EpCAM-coupled immunomagnetic beads, 73 % optical detection and dispensing efficiency (n = 5). 40 to 56.7 % of cells were recovered after complete isolation from 7.5 ml of untreated whole blood (n = 6). *
- Automated cfDNA enrichment from human plasma in a duplicated standard series was reproducible at 99.9 % (R2=0.9996) [unpublished data].
- The yield of extracellular vesicles (uEVs) isolated automatically from urine with our platforms for e.g. prostate cancer monitoring was approx. 2/3 higher than the yield of manually isolated uEVs. *
How can we work together and can you customize your process and biomarker targets?
In joint research and development work, we are happy to help you integrate target-specific immunoassays into our process automation. Our platform technology is very flexible.
* corresponding publications can be found on the right
 Fraunhofer Institute for Microengineering and Microsystems IMM
Fraunhofer Institute for Microengineering and Microsystems IMM